
- Ensure you are familiar with the medicine’s formulation, dose and frequency, safe dosing increments and adverse effects.
- Signs of opioid toxicity include drowsiness, myoclonic jerks, confusion/agitation, hallucinations, vivid dreams, cognitive impairment and respiratory depression. Pinpoint pupils are not a reliable sign of opioid toxicity in long-term opioid use.
- Confirm any recent opioid dose, formulation and frequency, and any other analgesics in use.
- Consider reduced doses in elderly, cachectic and debilitated patients and in patients with renal or significant hepatic impairment. Seek specialist advice.
- When changing dose or switching opioids, always monitor the patient for pain control and signs of opioid toxicity.
- Where a dose increase is intended, ensure the calculated dose is safe for the patient. Increase by no more than one third. Use caution in higher doses.
- When making a planned opioid switch, if there is no stated opioid equivalent, convert to the oral morphine equivalent and then to the chosen opioid.
- When switching opioids, a 25-50% reduction of the calculated dose of the new opioid is recommended, because tolerance to the initial opioid may not extend completely to other opioids. Review the new regimen at 24 hours and adjust accordingly, using caution at higher doses. For palliative care patients, ensure an “as required” opioid is prescribed.
- The addition of adjuvant analgesia may require reduction of the opioid dose.
- Before prescribing opioids or increasing doses, inform patients of risks, side-effects and potency of opioids. Patient information is available at https://niformulary.hscni.net/patient-area
Chronic pain (excluding palliative or cancer pain)
- In chronic pain, there is limited benefit from medicines, around 30% pain reduction.
- Do not initiate opioids for chronic primary pain. If a patient is already using an opioid, explain the lack of evidence, risks of continuing, and agree a shared plan for continuing, reducing or stopping (see NICE CG 193 1.2.11).
- Offer patients non-pharmacological strategies (see NICE CG 193 1.2.1-1.2.6). Signpost to resources on NI Formulary Chronic Pain and https://niformulary.hscni.net/patient-area.
- For chronic secondary pain refer to NICE guidance for the underlying condition and NI Formulary Chronic Pain.
Transdermal Opioid Conversion
- Transdermal patches are NOT appropriate when rapid titration of opioids is required e.g. acute pain. They are suitable in stable pain, if the transdermal route has a clear advantage.
- On first applying or increasing a patch, systemic therapeutic levels are not reached for at least 12. Do not adjust the dose until at least 48 hours have passed.
- On removal of a patch a reservoir of the opioid remains under the skin with levels falling by 50% (half-life) approximately every 18-24 hours. Consider this when switching to an alternative opioid or route.
- For information on starting, changing or stopping transdermal opioids refer to Palliative Adult Network Guidelines www.book.pallcare.info.
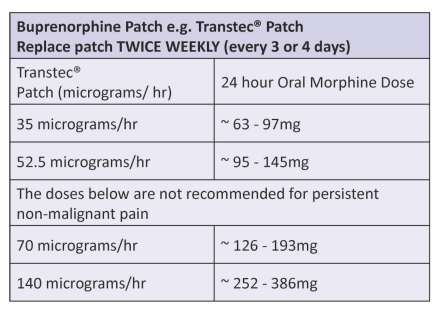
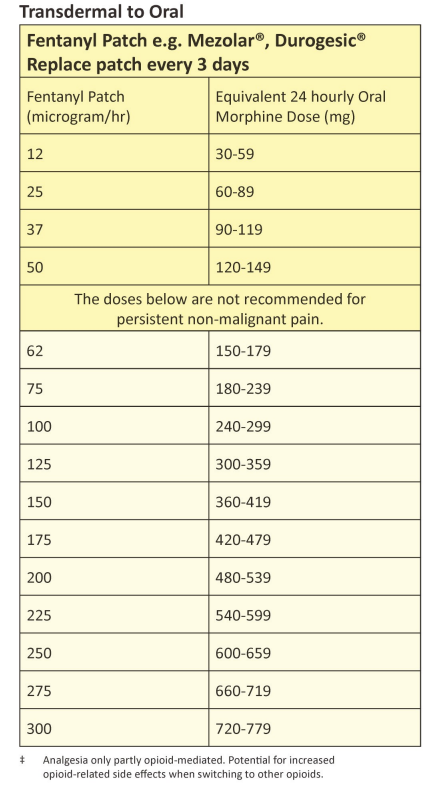
| Approximate equivalent doses of opioid analgesics for adult use – Read the above before using these equivalence tables |
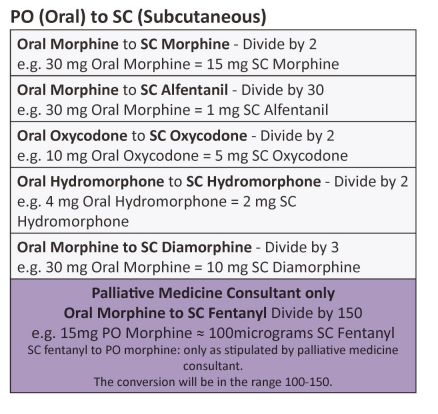
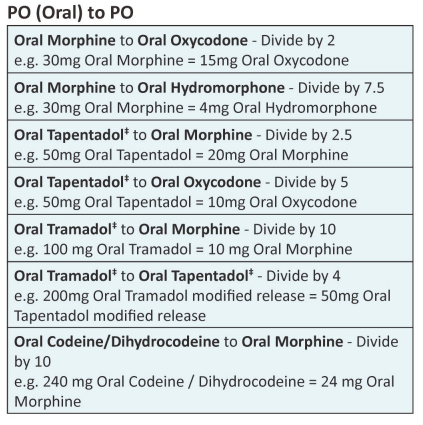
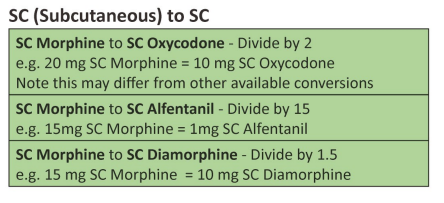
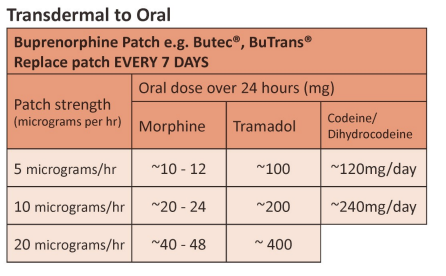
Developed by NI Palliative Care Pharmacy Group, Regional Palliative Medicine Group and Strategic Planning and Performance Group (SPPG) March 2023. For review March 2026.
Prescribing Opioid Analgesics
Morphine is the first line strong opioid.
Consider oxycodone as the first line choice in persistent renal impairment.
In hepatic impairment (without concomitant renal impairment) morphine is preferred.
In severe renal and/or hepatic impairment, or dialysis patients, seek specialist advice. Alfentanil, fentanyl or buprenorphine may be preferred.
Remember:
- Prescribe in mg or micrograms, not mL.
- Injected and oral routes are not equipotent. Specify one route for opioids. Never write PO/SC/IV in the route box.
- Prescribe in whole numbers. Avoid decimal points to reduce risk of error.
- Take care with sound alike names e.g. alfentanil and fentanyl.
- Know the product’s duration of action:
COMMON LONG ACTING (last 12 hours)
Oral modified release products
MST®, Zomorph®, Longtec®, Palladone SR®
COMMON SHORT ACTING (last around 4 hours)
Oral standard release products
Morphine sulfate oral solution, oxycodone oral solution, Shortec®, capsules, Sevredol® tablets, Palladone® IR capsules
Transdermal opioid patches
Mezolar®, Durogesic® : replace every 3 days.
Butec®, BuTrans® : replace every 7 days.
Transtec® , replace every 3 – 4 days.
Breakthrough Analgesia in Palliative Care
The standard regimen for strong opioids for breakthrough pain is usually one-tenth to one-sixth of the regular 24 hour dose, repeated every 4 hours as required. In palliative care, BNF supports prescribing every 2 to 4 hours as required, or hourly if pain is severe or in the last days of life {outside the product licence).
In persistent non-malignant pain, patients should not routinely require breakthrough analgesia except prior to events likely to cause pain e.g. dressing changes.
Disclaimer: Conversion ratios vary and these are an approximate guide only. They may differ from other published conversions but have been chosen to reflect best evidence and safety. Users are advised to monitor patients carefully for pain and side effects. Responsibility for the use of these recommendations lies with the healthcare professional(s) managing each patient. Seek specialist advice when necessary, especially at higher doses.
References
-
- British National Formulary Prescribing in Palliative Care bnf.nice.org.uk [accessed 24/11/22]
- Palliative Care Formulary 8th Edition (2022)
- Palliative Adult Network Guidelines (PANG) 2016. www.book.pallcare.info
- Health and Social Care Board NI Formulary www.niformulary.hscni.net
- Royal College of Anesthetists. Opioids Aware: A resource for patients and healthcare professionals. www.rcoa.ac.uk/faculty-of-pain-medicine/opioids-aware
-
- National Patient Safety. 2008. Reducing dosing errors with opioid medicines. NPSA/2008/RRR0057. Electronic Medicines Compendium 2022. Summary of Product Characteristics
- Tapentadol. Personal communication. Grunenthal June 2017
- Scottish Palliative Care guidelines www.palliativecareguidelines.scot.nhs.uk/guidelines/pain/pain-management [accessed 29/11/22]
- NICE CG 193 Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. Available at: https://www.nice.arg.uk/guidance/ng193 [accessed 25/01/23]
